Urine Analyzer

| Catalog Number | 58000 |
| OEM Catalog Number | 119 021224 021229 |
Semi-quantitative instruments analyze the chemical contents of urine with dry chemical methods, the analysis process uses strip urine with several test parameters of 8 and 14.
The instrument is easy to use. Innovatively designed and portable as well as can be integrated with other analytical urine instruments.
The PROLINE UA240 Urine Analyzer utilizes the principle of photoelectric colorimetry to measure the concentration of biochemical components in urine by detecting color changes resulting from the reaction between the urine sample and the reagent area on the test strip.
This Urine Analyzer operates in continuous (batch) mode, with the placement area for urine strips moves automatically, directing the strips to the analysis area. This process enables seamless and continuous sample reading.
| 1 | 6.7-inch touchscreen interface for operation |
| 2 | Infrared sensor to detect urine strips |
| 3 | Convenient and automatic urine strip tray |
| 4 | Built-in thermal printer |
| 5 | Waste container for collecting residual urine samples and used strips |
| 6 | Manual throughput: 240 tests/hour, normal: 600 tests/hour. |
| 7 | Reading options: 8 to 14 parameters. |
| 8 | Storage capacity of 2,000 data entries with LIS connectivity. |
| 9 | Reading time: <10 seconds |
| 10 | Stability: CV ≤ 1.0% |
| 11 | Light source: LED |
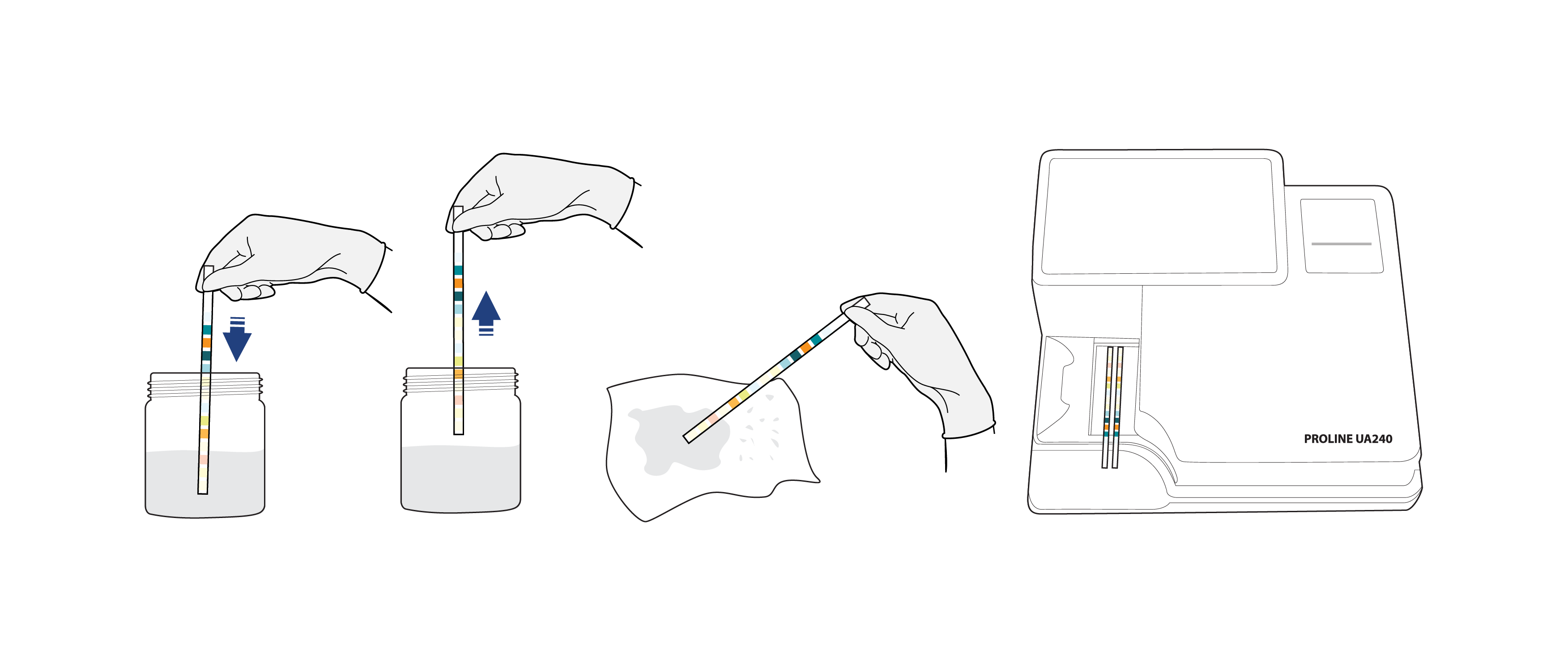
Quick Steps for Operating Urine Analyzer:
- Sample preparation. Prepare the urine sample in the sample container.
- Dip the urine strip into the sample container. Ensure that the entire reagent pad is fully immersed in the sample. Remove the strip and gently shake it inside the container.
- Clean excess sample residue on the strip with a dry cloth (avoid touching the reagent pad).
- Put the urine Strip on the tray sample. Make sure the strip is correctly positioned (with the reagent pad facing upward). Wait for the strip to move automatically. The results will appear automatically on the screen and printer within a few seconds.
| Operating type | Semi-automated |
| Work principle | Photoelectric colorimetry |
| Throughput | 240 test/hour |
| Detector | Photodiode LED |
| Storage capacity | 2000 datas |
| Parameters | 8 - 14 parameter |
| Wavelength | 470 nm, 525 nm dan 625 nm |
| Operational | Built in 6.7-inch touchscreen |
| Printer | Iternal thermal printer, paper size 57 mm |
| Repeatability | CV ≤1.0% |
| Stabilitas | CV ≤1.0% |
| Stability | 2 USB ports, 1 RS-232 port (external PC interface and bi-directional LIS/HL7) |
| Power consumption | Voltage: 100 V - 240 V
Frequency: 50/60 Hz Power: ≤45VA Fuse: T2AL250V |
| Dimensions | 39 cm (W) × 33 cm (D) × 20,3 cm (H) |
| Weight | 5 kg |
| Storage conditions | Temperature: -20℃ ~ 50℃
Humidity: ≤93% Atmospheric pressure: 76 kPa ~ 106 kPa |
| working environment conditions | Temperature: 10℃ ~ 30℃
Humidity: ≤80% Atmospheric pressure: 76 kPa ~ 106 kPa |
| Shelf Life | 5 Years |
| Service Life |
| Parameters | Abbreviation |
| Urobilinogen | URO |
| Bilirubin | BIL |
| Keton | KET |
| Creatinine | Cr |
| Blood | BLD |
| Protein | PRO |
| Microalbumin | MA |
| Nitrite | NIT |
| Leukocyte | LEU |
| Glucose | GLU |
| Specific Gravity | SG |
| pH | pH |
| Vitamin C | VC |
| Calcium | Ca |
| Catalog Number | Volume | |
|---|---|---|
| PROLINE URS11 | 24002 | 100 strip |
| PROLINE URS14 | 24003 | 100 strip |
- PROLINE UA240 Urine Analyzer
- Doos
- Manual Book
- Quick Guide
- Stater Kit (reagen 1 pack)
- Thomas L. Alanine aminotransferase (ALT), Aspartate aminotransferase (AST). In: Thomas L, editor. Clinical
Laboratory Diagnostics. 1st ed. Frankfurt: TH-Books Verlagsgesellschaft; 1998. p. 55-65. - Moss DW, Henderson AR. Clinical enzymology. In: Burtis CA, Ashwood ER, editors. Tietz Textbook of
Clinical Chemistry. 3rd ed. Philadelphia: W.B Saunders Company; 1999. p. 617-721. - Bergmeyer HU, Horder M, Rej R. Approved Recommendation (1985) on IFCC Methods for the Measurement
of Catalytic Concentration of Enzymes. L Clin. Chem. Clin. Biochem 1986; 24: 481-495. - Bakker AJ, Mücke M. Gammopathy interference in clinical chemistry assays: mechanisms, detection and
prevention. ClinChemLabMed 2007;45(9):1240-1243. - Guder WG, Zawta B et al. The Quality of Diagnostic Samples. 1st ed. Darmstadt: GIT Verlag; 2001; 14-5.
- Young DS. Effects of Drugs on Clinical Laboratory Tests. 5th ed. Volume 1 and 2. Washington, DC: The
American Association for Clinical Chemistry Press 2000.
- Brochure : INA |
Reach our team to know more about other products and order information.
- WA. +62 815 1359 2626
- E. marketing@proline.co.id
Please reach our Technical Support team for further help with product specification, services, and other technical documents.
- WA. +62 817 9324 884
- E. technical.support@prodis.co.id